Packaging material made from polyethylene terephthalate (PET)
DLG Expert report 4-2016
Author:
- Dr. Frank Welle, Fraunhofer Institute for Process Engineering and Packaging IVV, Giggenhauser Strasse 35, 85354 Freising, Germany, Welle@ivv.fraunhofer.de
Contact:
- Dr.-Ing. Annette Schmelzle, DLG Competence Center Food, A.Schmelzle@DLG.org
In cooperation with the DLG Food Technology Committee.
Introduction
Plastic bottles made from polyethylene terephthalate (PET) have gained a very high share of the market in the past decade. They are increasingly replacing glass bottles, cans and also beverage cartons. PET bottles are largely used as non-returnable bottles. However in Germany the share of returnables in the total number of PET bottles has stabilised at around 10%.
The first polyesters were produced in the 1930s. At that time polyester was chiefly used to make synthetic fibres, which were marketed e.g. under the trade names Trevira® and Diolen®. A large share of the PET material produced worldwide is still used for fibres today –fleece pullovers for example are made from PET. A little later PET could also be used to produce packaging films. PET films also served as a carrier for reels of film or magnetic tapes. Finally, in the 1970s, the industry succeeded in making bottles from PET. At the end of the 1980s the first PET bottles were launched on the market in Germany. They were initially only used for sweet beverages, but gradually PET bottles also established themselves as packaging for mineral water.
PET is made from terephthalic acid (a dicarboxylic acid) and ethylene glycol (a dialcohol). Both substances combine to form long polymer chains. Water results as a product of reaction. The polymerisation reaction proceeds in several stages. First a „pre-polymer“ is produced, which is then polymerised further in the melt to longer chains. Like most polymerisation reactions, this reaction too needs a catalyst. For use in PET bottles, in a further production stage the polymer is then heated in granular form for several hours. This results in a polyester polymer which displays the right properties for producing PET bottles.
PET as packaging material
PET is a colourless, transparent polymer that is almost unbreakable and can be recycled well. These good properties led to broad distribution of PET as a packaging material for beverages. By contrast with beverage cans or cartons, the transparency allows the beverage to be seen. The unbreakable nature of PET is an advantage over glass bottles. Moreover PET is distinctly lighter than glass.

Nowadays disposable PET bottles only weigh about 20 g to 30 g. A glass bottle with a comparable content would weigh around 25 times more. Returnable PET bottles are a little heavier due to their somewhat thicker walls and more robust base. The low weight has a positive effect on transport costs. Moreover disposable PET bottles are conveyed to the fi lling plants as preforms and only blow-moulded to fi nished bottles there. This makes it possible to greatly improve the payload of the transport vehicles.
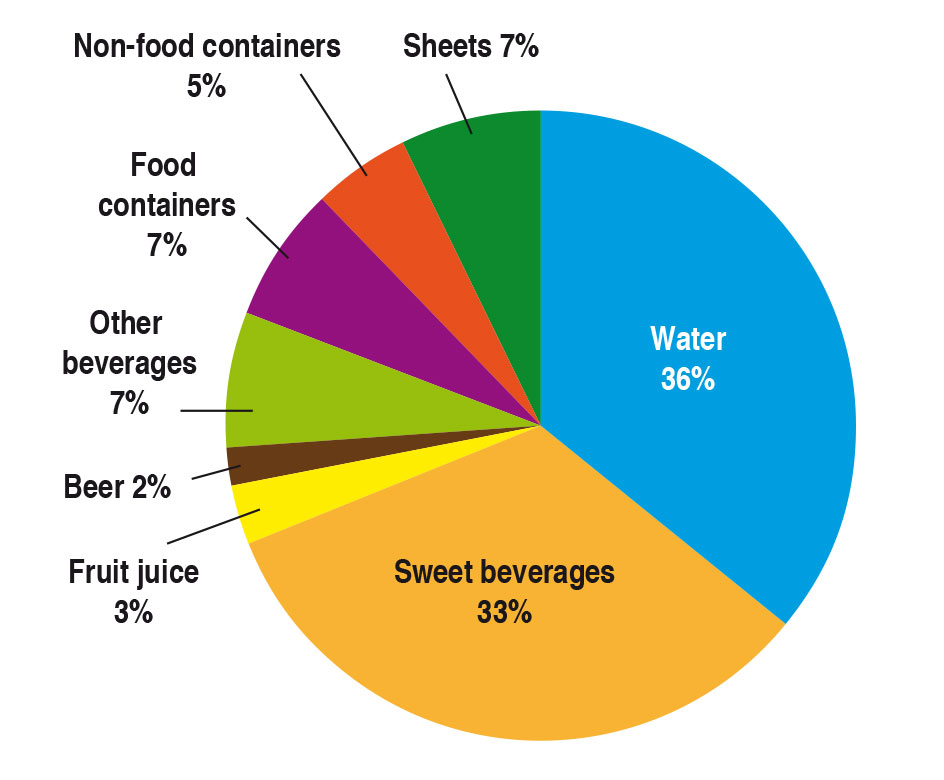
In the packaging sector PET is used to make films, trays and bottles. Beverage bottles dominate production for the packaging sector. In 2014 about 80 % of the material was used to package beverages, and almost 70 % of this for mineral water and sweet beverages (Figure 2).
PET bottles are produced in a two-stage process. The PET granules are melted at about 280 °C and processed to create a preform. This preform already possesses the later closure thread, but is small and, as already mentioned, can be transported well. Shortly before the filling process the preform is heated up again to about 120 °C and blow-moulded into its fi nal bottle form. In the case of disposable PET bottles this step is performed directly at the fi lling plant. Returnable PET bottles are delivered to the filling plant as fi nished bottles. The PET material is partly crystallised by the „stretch blow moulding process“. Although a production process with partially crystalline sectors reduces transparency a little, it improves the stability of the PET bottles and the barrier against oxygen and carbonic acid. Completely crystalline PET is opaque and is used, for example, for microwave trays.
Conformity of PET bottles with food law
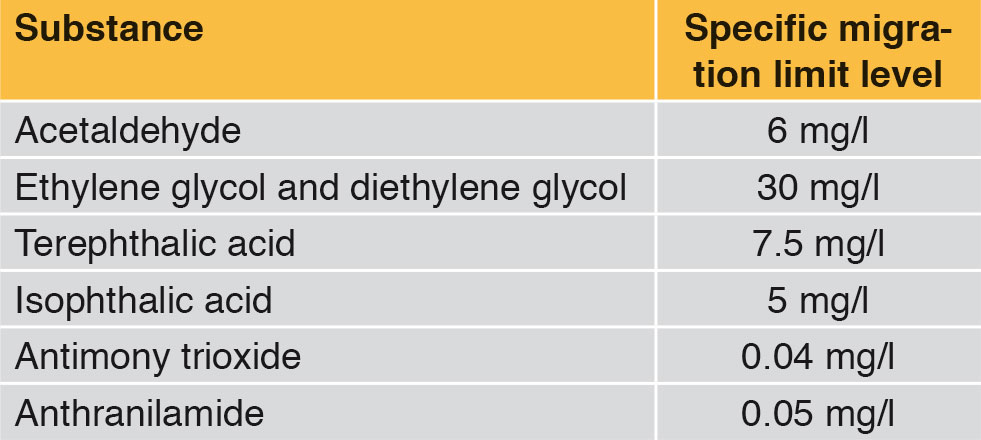
Interactions occur between all packagings and the food they contain. For example, oxygen can infi ltrate from the exterior into the packaging, or small quantities of carbon dioxide can leak through the bottle wall. Moreover migration of polymer ingredients from the packaging into the beverage can occur. This migration should be reduced to a minimum for the sake of consumer health protection. In addition the raw materials deployed, such as for example the monomers ethylene glycol and terephthalic acid, or the catalyst antimony trioxide and other substances used, have been given limit levels. The substance anthranilamide authorised as an additive for PET bottles also has a specific migration limit value (Table 1). Alongside these specifi c limit levels, the total quantity of all substances that are allowed to migrate into the food is limited.
Migration of substances from the packaging into the beverage cannot be ruled out for plastics in general. However, by comparison with other plastics PET has very inert properties. Accordingly only very small amounts of substances migrate from the packaging into the beverage. Despite this the beverage filling plant must analyse and appraise the migration from PET bottles and keep the appropriate certifications. As a general rule, migration is all the lower the shorter the storage period and the lower the temperature.
Total migration
The limit level for the total of all substances that are allowed to migrate into the food is 10 mg per 1 dm2 packaging area. The values measured for total migration of PET bottles are normally around 0.1 mg per dm2, in other words lower than the limit level by a factor of 100 [1].
Monomers
The migration of the two monomers ethylene glycol and terephthalic acid is also very low. It is virtually impossible to exceed the legal limit levels for the migration of monomers such as ethylene glycol and terephthalic acid from PET bottles in practice [1].
Catalyst antimony
Antimony trioxide is used as a catalyst in polymerising PET. In principle, catalysts remain behind in the PET after polymerisation. That is why migration of the catalyst residues must be checked. Alternative catalysts have also been developed, mainly on the basis of the elements titanium or germanium. However, these alternative catalysts have not yet become established in PET bottles.
The migration of antimony is higher in PET bottles than in glass bottles, which do not contain antimony. For this reason there have repeatedly been reports in the media in recent years of migration levels being higher in PET bottles than in glass bottles. Like all substances used to produce PET, antimony too is subject to legal regulations in Europe. A maximum of 0.04 mg antimony may migrate from a PET bottle into one litre of a beverage. Drinking water limit levels for antimony are much lower than the migration limit level from packagings. In Europe, for example, a maximum of 0.005 mg antimony per litre of drinking water may be detectable. The distinctly lower drinking water limit level by comparison with packagings is due to the fact that drinking water is additionally used for cooking and washing. The legislature takes this circumstance into account by setting a lower limit level.
The migration of antimony from PET bottles into the beverage is also very low. The limit level of antimony in PET bottles cannot be exceeded within the stated shelf life of the beverage, even if the bottles are kept in storage for years at higher temperatures (e.g. in tropical climates). Nor can the lower drinking water limit level be exceeded [2]. The concerns voiced in newspaper reports are therefore unfounded. However, they rightly point out that migration of antimony into beverages is higher with PET bottles than with glass bottles.
Acetaldehyde
Acetaldehyde is a byproduct of PET production. It is formed when PET is melted at high temperatures, for example in the production of PET performs. However, acetaldehyde occurs not only in PET.
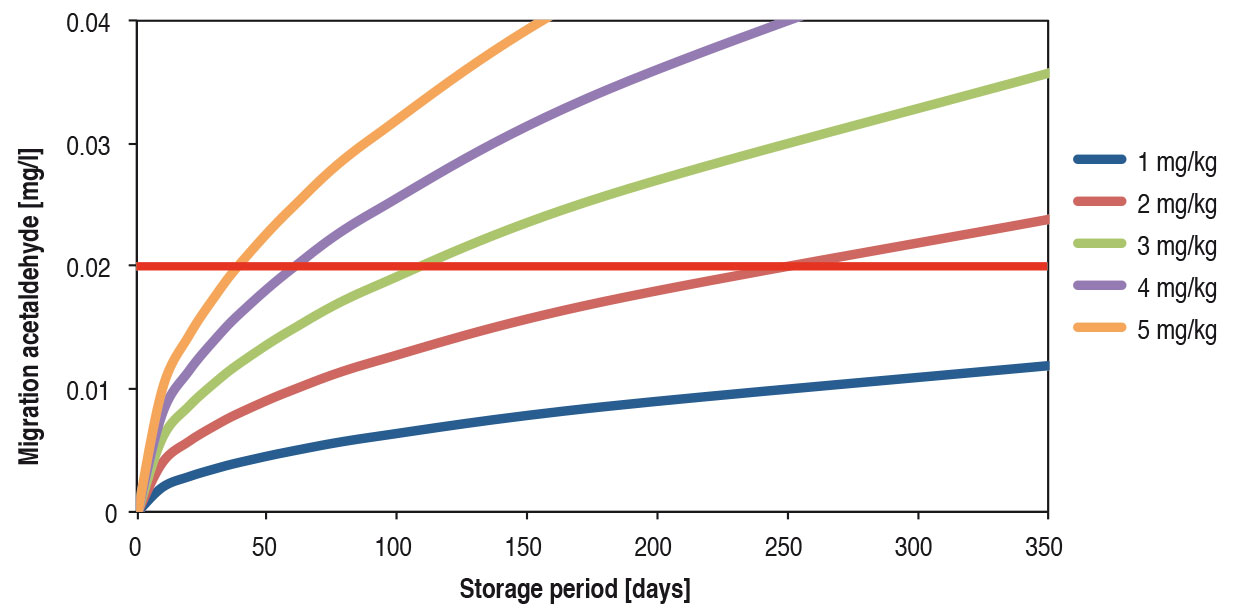
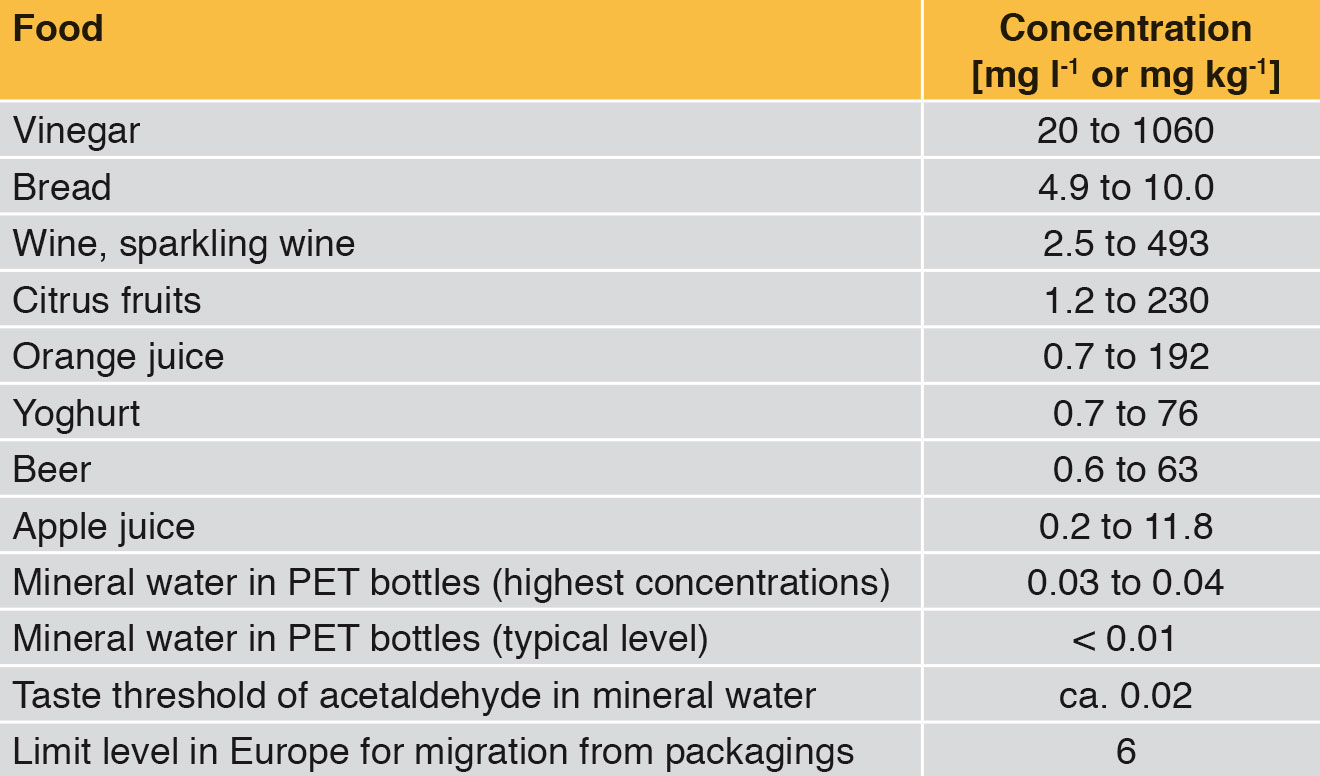
For example many beverages and foods contain slight quantities of acetaldehyde by nature (Table 1). That is why traces of acetaldehyde in mineral water give no rise to health concerns. However, acetaldehyde creates a sweetish off-flavour in mineral water. From a concentration of 0.02 mg acetaldehyde per litre of mineral water upwards, the average consumer can certainly perceive the taste of acetaldehyde. This off-flavour in mineral water is of course undesired. Together with the mineral wells / springs, the producers of PET bottles have therefore optimised PET bottle production methods and thus reduced the migration of acetaldehyde. The concentration of acetaldehyde in the PET bottle wall is typically 1 to 2 mg/kg. This allows a shelf life of around 9 months (Figure 3).
This taste impairment through acetaldehyde does not play any role in the case of sweet beverages, juices and beer, as the concentration of acetaldehyde in the actual beverage is already distinctly higher than the migration from the PET bottle (Table 2).
Anthranilamide
Slight quantities of acetaldehyde can migrate into the mineral water and cause a sweetish off-flavour there. In order to prevent this, the additive anthranilamide is used in the production of PET bottles. This binds acetaldehyde chemically and thus reduces the concentration of acetaldehyde in the PET bottle wall. Consequently there is lower migration of acetaldehyde into the mineral water, so that the flavour threshold of acetaldehyde in mineral water is no longer exceeded within the shelf life period (Figure 3). The additive anthranilamide used for this purpose can itself migrate into the mineral water. However the concentrations of anthranilamide in the mineral water remain below the legally authorised migration limit level [4]. No anthranilamide is used in PET bottles for sweet beverages, juice and beer.
Does PET contain plasticisers?
Plasticisers are added to various plastics in order to influence the material properties of the plastic. Generally these are phthalic acid esters or adipic acid esters. Plasticisers serve to make plastics „softer“. This is necessary for instance in the case of polyvinyl chloride (PVC). In the case of PET, however, it is desirable for the bottles to be hard and stiff. They can then be made thinner and this reduces the bottle weight. Moreover the bottles can then be stacked better on the pallets. That is why it would make no sense to add plasticisers to the PET bottles. Consequently PET bottles do not contain plasticisers.
The name terephthalic acid used as a monomer sounds very similar to the source material used for plasticisers – phthalic acid. That is why a connection is often erroneously made between PET and plasticisers. In chemical terms, however, plasticisers are small molecules that push their way in between the long polymer chains and so make the polymer more flexible („softer“). PET on the other hand is a very large „macro molecule“.
Does PET contain bisphenol A?
Bisphenol A is one of the monomers of polycarbonate (PC). Bisphenol A and polycarbonate are, however, not used in the production of mineral water bottles. One application of polycarbonate was baby bottles. Here polycarbonate provides the necessary material properties. For example these bottles can be repeatedly boiled and thus sterilised, which would not be possible with PET bottles. Baby bottles made of polycarbonate can thus give off traces of the monomer bisphenol A. Bisphenol A is classified as a hormone-active substance. In order to fulfil its duty of care, however, the legislature has by way of precaution prohibited the use of bisphenol A for producing baby bottles. No bisphenol A is used in the production of PET bottles.
Are there any hormone-active substances in PET bottles?
In the year 2009 there were reports in the media that hormone-active substances make their way into mineral water from PET bottles. Hormone-active substances have a similar effect on humans as the natural hormone estradiol. That is why the concentrations are expressed in estradiol-equivalents. Peak values of 75 ng (nanogram, a billionth of a gram) estradiol-equivalents were reportedly detected in a mineral water in PET bottles. However, the substances responsible for this were not found. The German Federal Institute for Risk Assessment (BfR) promptly published an evaluation in which it concluded that the results did not allow any conclusions as to the origin of such hormonally-active substances from PET bottles [5]. Mineral water samples were subsequently examined in a number of studies conducted by national surveillance laboratories [6,7]. The concentrations found were at most 5 pg (picogram, one billionth of a gram) per litre of mineral water, in other words around a factor of 15000 lower than the originally discussed 75 ng. An overview of the literature on substances with an endocrine effect in mineral water is provided in the literature. [9].
The World Health Organization WHO [8] derives an admissible daily dose of estradiol of 3 μg (microgram, one millionth of a gram) per person and day. This level relates to a person with a body weight of 60 kg. The concentrations found in the range of 5 pg estradiol-equivalents per litre of mineral water are accordingly below this by a factor of around 600,000. Consequently the media reports from the year 2009 are unfounded. The high concentrations of estradiol-equivalents could not be confirmed by subsequent studies and the concentrations do not represent any health hazard for consumers.
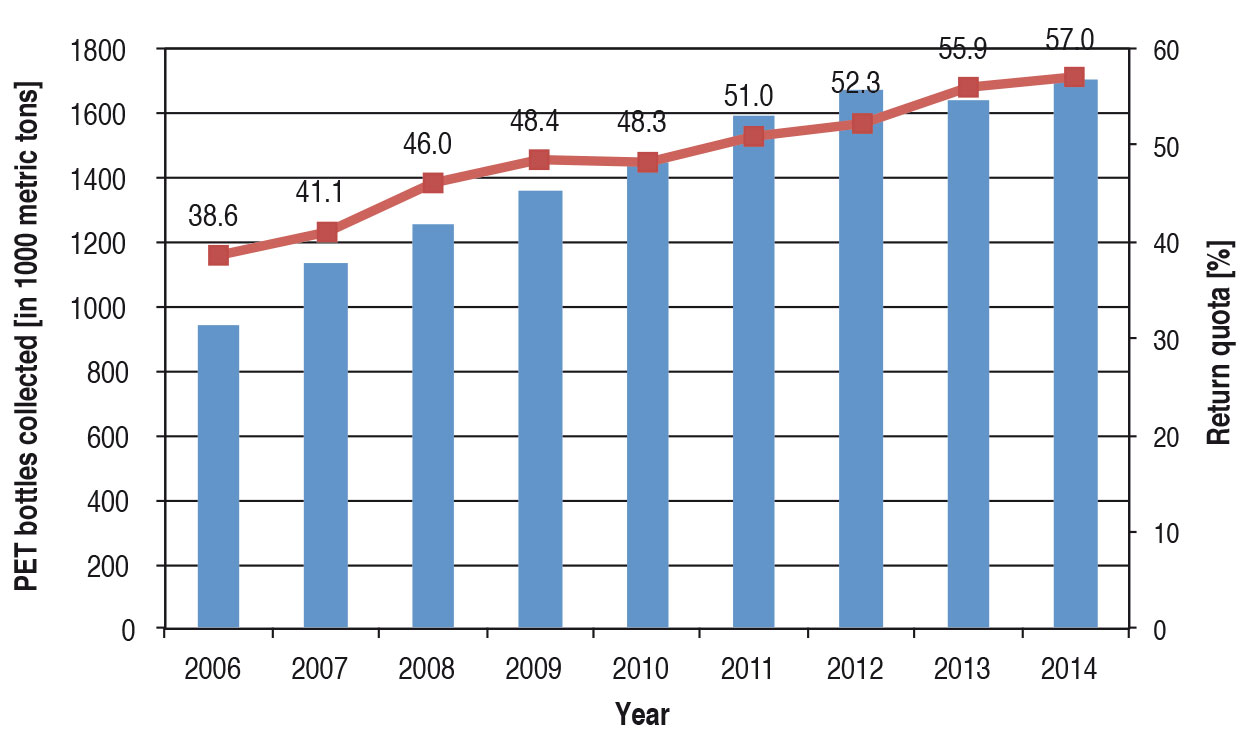
Recycling of PET bottles
PET bottles are in every respect a special case in the recycling of packagings. They represent a large share of packaging materials generated and it is very easy to automate sorting. Consequently large quantities of used (post-consumer) PET bottles occur that can be recycled. The volume collected in Europe has risen steeply in recent years. In the year 2014 altogether 1.7 million metric tons of used PET bottles were collected and passed on for recycling throughout Europe (Figure 4). This means that in Europe around 57 % of all PET bottles on the market are collected again and recycled. In some countries such as Germany, Iceland, Norway and Switzerland, the return quota is around 90 %.
<p>PET can be 100 % recycled. In other words it can be melted down again almost as often as desired. In PET recycling the bottles are first crushed and the labels and caps are removed. After a washing process the recycled material is available for use as source material for high-grade products, for example for fleece pullovers, sleeping bags or insulation materials. In the last two decades, the constant further development of recycling processes has also led to recycled PET material that can be integrated into new PET bottles again [9]. Accordingly a new PET bottle results from a used PET bottle. However, this calls for additional cleaning steps known as „super-clean“ recycling processes. Super-clean processes are subject to authorisation by the European Food Safety Authority (EFSA). This ensures that only recycling firms with an effective cleaning process and a quality assurance concept are allowed to produce „super-clean“ recycled material that may then be integrated into the PET bottles. Under the aspect of migration, these recycled materials can no longer be distinguished from new PET products. PET bottles with recycled material are therefore just as safe as new PET bottles. The recycled share in PET bottles can be up to 100 %. PET bottles with 50 % recycled material are standard today.</p>
Conclusion
Thanks to its very good properties such as transparency, recycling capability and low migration of substances into the food, the use of PET as a packaging material has become established with a very large share of the market, in particular for beverages. And this positive trend shows no sign of stopping. Market research institutes predict worldwide growth of almost 4 % a year for PET packagings. Negative reports on PET, regarding for instance plasticisers, bisphenol A and hormone-like substances that have reportedly been found in PET bottles, have not been confi rmed. PET is therefore a good packaging material with a successful future.
Literature:
- [1] A. Störmer, R. Franz, F. Welle, New concepts for food Law compliance testing of polyethylene terephthalate bottles, Deutsche Lebensmittel-Rundschau, 2004, 100(2), 47-52
- [2] F. Welle, R. Franz, Migration of antimony from PET bottles into beverages: Determination of the activation energy of diffusion and migration modelling compared to literature data, Food Additives and Contaminants, 2011, 28(1), 115-126.
- [3] L. M. Nijssen, C. A. van Ingen-Visscher, J. J. H. Donders, VCF (Volatile Compounds in Food) database, TNO, Zeist, The Netherlands, 2009. Available online http://www.vcf-online.nl
- [4] R. Franz, M. Gmeiner, A. Gruner, D. Kemmer, F. Welle, Diffusion behaviour of the acetaldehyde scavenger 2-aminobenzamide in polyethylene terephthalate for beverage bottles, Food Additives and Contaminants, 2016, 33(2), 364-372.
- [5] Hormonell wirkende Substanzen in Mineralwasser aus PET-Flaschen, Information Nr. 006/2009 des BfR vom 18. März 2009 zu einer Studie der Universität Frankfurt am Main.
- [6] B. J. Brüschweiler, P. Y. Kunz, Hormonaktive Substanzen in abgepacktem Mineralwasser Schweizerisches Bundesamt für Gesundheit BAG Bulletin 14/11 2011.
- [7] K. Bopp, B. Kuch, M. Roth, Hormonelle Aktivität in natürlichen Mineralwässern? Deutsche Lebensmittel- Rundschau, 2010, 106(7), 489-500.
- [8] Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA): Estra-1,3,5(10)-triene-3, 17-beta-diol, CAS No. 50-28-2, available online: http://apps.who.int/ipsc/database/evaluations/chemical.aspx?chemID=1835
- [9] F. Welle, Hormone in Mineralwasser? Eine kritische Analyse, Deutsche Lebensmittel-Rundschau, 2014, 110(4), 162-166.
- [10] F. Welle, Twenty years of PET bottle to bottle recycling – An overview, Resources, Conservation and Recycling, 2011, 55(11), 865-875.
Contact:
Dr.-Ing. Annette Schmelzle, DLG Competence Center Food, A.Schmelzle@DLG.org
In cooperation with the DLG Food Technology Committee.